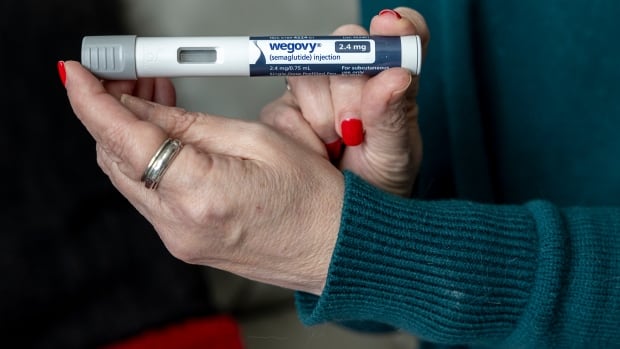
The popular weight-loss drug Wegovy has been available in Canada since early May, but anybody hoping to use it will have to shell out about $400 a month.
One advocate says that lack of coverage will hurt the country in the long run.
“There is a cost of inaction in terms of not providing care, right? More people showing up to emergency departments with complications … or increased medication costs for all the other co-morbidities, like diabetes, high cholesterol,” said Dr. Sanjeev Sockalingam, scientific director of Obesity Canada, an organization dedicated to improving the lives of people living with obesity.
Wegovy is a weekly injection made by Novo Nordisk, the Danish company who make the diabetes drug Ozempic. Both drugs have the same active ingredient — semaglutide — but Wegovy is a higher dose.
Approved by Health Canada more than 2 years ago
Health Canada approved Wegovy to treat people diagnosed with obesity in November 2021, but Canada’s drug agency recommended provinces and territories not reimburse for its use. The Canadian Agency for Drugs and Technologies in Health (CADTH) said Wegovy was effective and had an acceptable side effect profile, but that it was unclear whether it reduced co-morbidities and improved quality of life.
Sockalingham told host Matt Galloway of CBC Radio’s The Current that Wegovy should be covered by provincial drug plans.
“As we continue to not have access, we have more people living with obesity, developing complications … and living with impaired quality of life,” he said.
LISTEN | Wegovy costs $400 a month. Should provinces pay?
The Current22:45Wegovy costs $400 a month. Should provinces pay?
He said even beyond weight loss there is evidence that the drug helps some people with pre-existing cardiovascular disease. Studies are also being done on whether it helps sleep apnea and liver inflammation, he said.
Sockalingham said it makes sense to conduct a cost-benefit analysis of a drug that costs thousands a year, but that obesity is widespread enough in Canada to justify the expense.
“If I were to say to you that we had a cancer medication that seemed to work better than other agents, had better health benefits long-term, and we told patients living with cancer we would not provide coverage for that, we probably would have an uproar,” he said.
‘Affordable and accessible to all Canadians’
The founder of a support group for people living with obesity told The Canadian Press last month that the high cost of Wegovy raises the issue of equitable access.
“Many in our community, and especially those in the lower socioeconomic bracket, they find these treatments are financially out of reach,” said Priti Chawla, executive director of Obesity Matters.
“It’s essential that we work towards making Wegovy affordable and accessible to all Canadians who need it.”
However, at least one expert says Canada should wait for more evidence before covering a drug that is expensive and taken indefinitely.
Dr. Joel Lexchin, retired physician and professor emeritus at the school of health policy and management at York University, said data on the drug’s long-term effectiveness is too limited. He’d like to see more studies before there’s blanket coverage.
Lexchin said the money could be better spent on improving public education or access to high-quality food. In the meantime, he’d prefer to see Wegovy used on a restricted basis while more data comes in.

“And preferably, we also should need that evidence publicly funded, rather than coming from drug studies that are funded by drug companies,” he said.
Wegovy treats obesity on the individual level when it needs to be dealt with on a societal level, he said.
Lexchin said he expects private insurers to cover the drug, as is typical, but that they may put a time limit on the coverage.
Chronic medical condition
Wegovy arrived after extensive marketing of Ozempic and a social media-driven surge in demand for off-label use of that drug for weight loss. Experts say it’s critical that prescribers, including family doctors, ensure Wegovy is only given to patients who meet specific obesity criteria.
Sockalingham says obesity should be treated like diabetes and other chronic diseases. He stressed that using Wegovy for obesity is about treating a medical condition, not cosmetic changes.
“Many people still believe that this is a eat-less, exercise-more phenomenon. ‘This is about willpower. It’s not a medical condition.’ And so the validity of obesity as a chronic medical condition continues to be questioned, despite evidence, despite the neurobiology of obesity and how we understand obesity in 2024, despite treatments that really show benefits in terms of outcomes and weight loss,” he said.
Canada’s drug agency declined an interview, but said in a statement that new trial results have been published since its initial review of Wegovy in 2022. Nova Nordisk is free to make a new submission, the statement said.