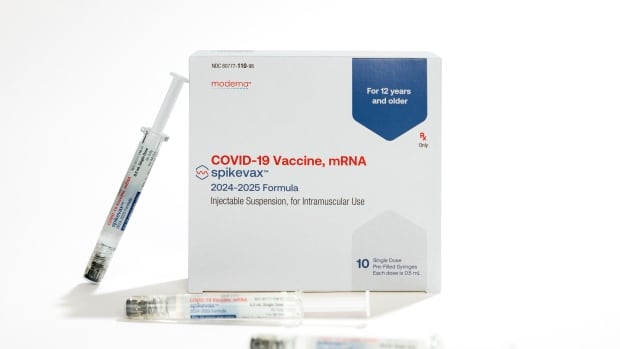
Health Canada authorized Moderna’s updated COVID-19 vaccine on Tuesday to roll out in fall immunization campaigns for those aged six months and older.
The federal vaccine portal lists approval of Moderna’s product.
A spokesperson for Moderna said it will start delivering the product to the Public Health Agency of Canada in the next day or two and expects “a robust supply will be available in time for provincial and territorial vaccination campaigns, but specific timing depends on provinces.”
The pharmaceutical company said the vaccine targets the KP.2 variant, one of the latest offshoots of Omicron.
The Omicron variant of concern set off a massive wave of infections worldwide starting in November 2021.
In August, the federal government sent a notice to health professionals saying the previous COVID-19 vaccines targeting an earlier variant would no longer be available as “part of regulatory and supply management best practices, consistent with the approach to annual influenza vaccines.”
Health Canada is also currently reviewing, on an expedited basis, submissions from Pfizer and Novavax for planned fall immunization campaigns aiming to protect people from severe hospitalizations and deaths from COVID-19.
Early research suggests a concerning percentage of recovered COVID-19 patients have been left with a chronic respiratory condition called pulmonary fibrosis.
The COVID-19 activity level is currently “moderate,” according to the federal map of viral activity based on available wastewater data. Wastewater testing offers an early indicator of when respiratory viruses are on the rise, doctors and epidemiologists say.
Health officials recommend updated vaccines since the virus that causes COVID-19 continues to mutate or change, and protection from infection and immunization wanes over time.